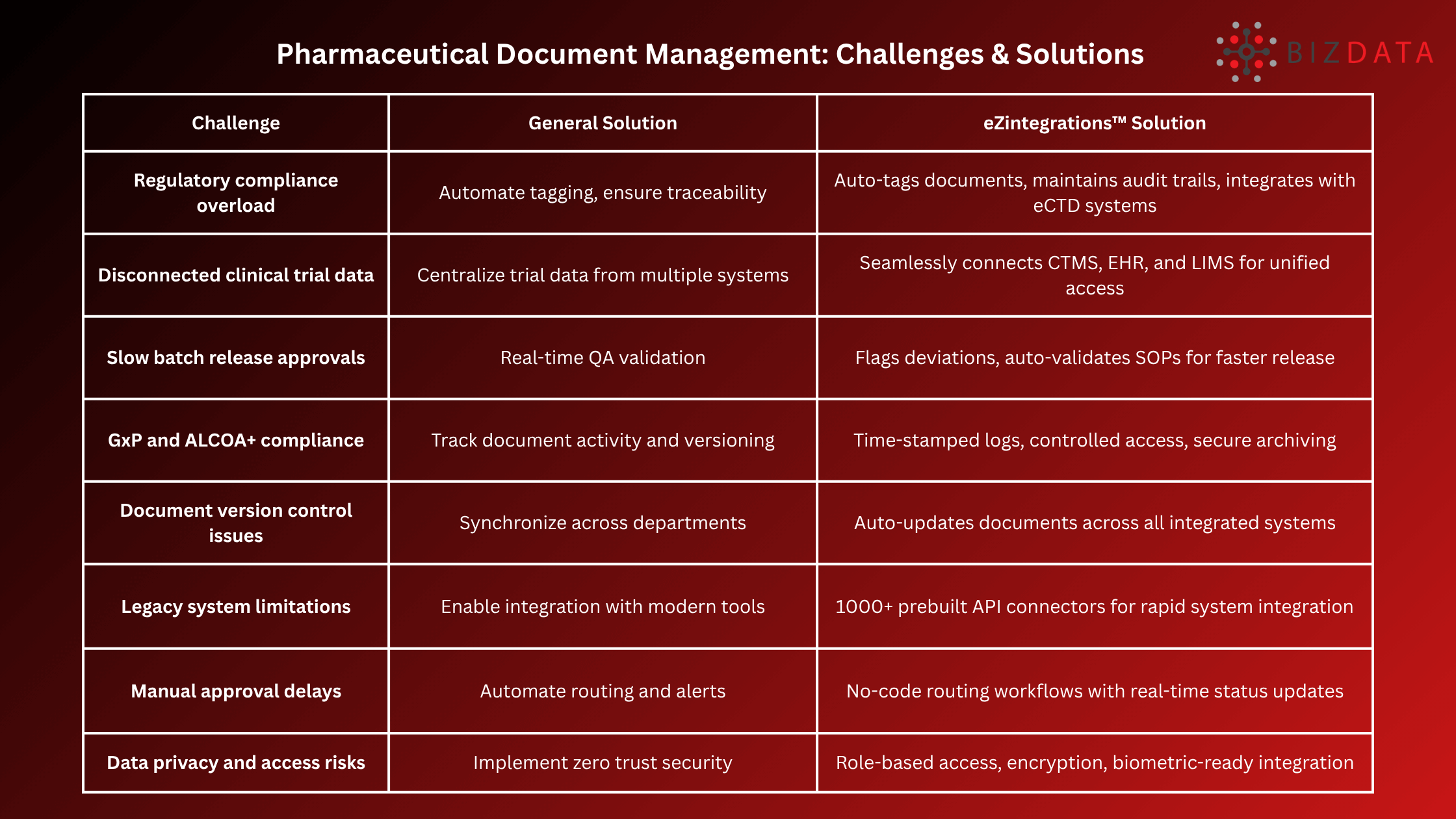
The pharmaceutical industry is drowning in documentation. From regulatory filings and clinical trial records to manufacturing protocols and patient data, the sheer volume of documents is staggering.
According to a Deloitte report, pharmaceutical companies spend up to 25% of R&D time managing documents manually, costing the industry billions annually. More than 70% of pharma data is unstructured, trapped in PDFs, scanned images, and handwritten records.
Manual handling of these documents not only slows down innovation but increases the risk of non-compliance, data loss, and human error. In an industry where one misstep can cost millions or worse, impact patient safety inefficiency is unacceptable.
AI-powered pharmaceutical document management. By leveraging platforms like eZintegrations™ and Goldfinch AI, organizations can automate and intelligently manage the entire document lifecycle, transforming bottlenecks into streamlined workflows.
This blog is for tech professionals, digital transformation leaders, and pharma IT teams who are exploring intelligent document automation to meet evolving compliance demands and accelerate go-to-market timelines.
Pharmaceutical document management refers to the systems and processes used to capture, store, retrieve, manage, and track documents across the drug development and delivery lifecycle. These include:
In a highly regulated industry, maintaining version control, audit trails, and compliance with GxP guidelines is critical. Traditional document management systems, however, fall short when dealing with unstructured formats or scaling for enterprise needs.
AI-powered pharmaceutical document management goes beyond digitization. It uses advanced technologies like:
These technologies enable context-aware automation, reduce the need for manual review, and make pharma operations audit-ready.
The pharmaceutical industry deals with diverse documents, such as Drug Master Files (DMF), New Drug Applications (NDA), Clinical Trial Protocols and Reports, Investigator’s Brochures, and more. Each document type comes with its own unique format and content structure, making it difficult to extract relevant data accurately and efficiently.
Poor formatting in pharmaceutical documents can impede data extraction processes. Inconsistent layouts, tables, and text styles often hinder accurate information retrieval, resulting in time-consuming manual interventions.
Many pharmaceutical documents span multiple pages, making it challenging to extract and process data. Manually handling such documents is laborious and prone to errors, slowing down the overall document understanding process.
Timely extraction of crucial information from clinical trial documents, such as patient records, informed consent forms, and adverse event reports, is essential for effective healthcare decision-making. Traditional manual methods lag in providing real-time data extraction, creating delays and hindering prompt action.
Pharmaceutical documents may contain handwritten information that poses additional difficulties for data extraction. Automated systems must be capable of accurately processing and understanding handwritten content to ensure reliable data extraction.
Maintaining document quality is paramount in the pharmaceutical industry. Poor quality documents, including those with faded text, smudged ink, or illegible handwriting, greatly hinder the accuracy and reliability of data extraction.
Pharmaceutical documents often contain sensitive personal and medical information. Ensuring privacy and security while handling this data is of utmost importance to complying with privacy laws and regulations.
The pharmaceutical industry deals with many documents, ranging from clinical trial reports to quality control records.
Extracting and processing data efficiently while scaling up operations is crucial to meet industry demands.
AI-Powered Use Cases in Pharmaceutical Document Management
eZintegrations™ AI Document Understanding revolutionizes document understanding in the pharmaceutical industry. By incorporating cutting-edge technologies such as Natural Language Processing (NLP) and machine learning, it offers the following solutions:
Through automated data extraction, eZintegrations™ AI Document Understanding accelerates the process, resulting in time savings and improved data accuracy. Medical experts and researchers can make informed decisions based on extracted data, enabling the speedy development of life-saving therapies.
eZintegrations™ AI Document Understanding utilizes document-specific templates to handle content variations effectively. By understanding the structure and format of each document type, it can extract relevant data accurately, even from documents with complex layouts.
The system’s advanced algorithms enable intelligent recognition of various document elements, including tables, text styles, and pagination. This capability ensures the accurate extraction and interpretation of information, regardless of document length or formatting issues.
eZintegrations™ AI Document Understanding leverages its AI-powered handwriting recognition capabilities to accurately process and understand handwritten content. As a result, critical data from pharmaceutical documents, such as patient records or handwritten annotations, can be reliably extracted and utilized for analysis and decision-making.
eZintegrations™ AI Document Understanding incorporates advanced image processing techniques to assess and improve document quality. It can detect and address issues such as faded text, smudged ink, and illegible handwriting to ensure high-quality data extraction and reliable document understanding.
eZintegrations™ AI Doc Understanding understands the importance of sensitive data handling in the pharmaceutical industry. To maintain compliance with privacy laws and regulations, the system implements robust security protocols to safeguard confidential information during the data extraction and processing stages.
With its ability to handle large volumes of documents, eZintegrations™ AI Document Understanding ensures scalability and efficiency in document understanding processes. It can rapidly extract and process data from various types of pharmaceutical documents, such as Clinical Trial Protocols, Good Manufacturing Practice (GMP) Documentation, and Regulatory Compliance Documents, allowing for streamlined operations as the industry expands.
eZintegrations™ AI Document Understanding seamlessly integrates with enterprise resource planning (ERP) systems, enabling efficient data transfer and consolidation. This integration eliminates manual data entry, reducing errors, and enhancing overall financial management and back-office tasks.
AI-powered pharmaceutical document management isn’t a futuristic concept. It’s a present-day necessity. By automating the most complex and error-prone parts of pharmaceutical documentation, AI tools like eZintegrations™ and Goldfinch AI help teams move faster, reduce risk, and focus on what matters: patient outcomes and innovation.
eZintegrations™ AI Document Understanding addresses key challenges in pharmaceutical document processing using advanced NLP, machine learning, and intelligent data recognition. It streamlines data extraction across varied formats, improves accuracy, ensures compliance, and enhances scalability ultimately driving efficiency and better patient outcomes.
In an industry that can’t afford delays or compliance missteps, automation is your competitive edge.
Book your free demo of eZintegrations™ today and explore the future of intelligent pharma documentation.
1. What is pharmaceutical document management?
It’s the structured process of handling clinical, regulatory, quality, and manufacturing documentation throughout a drug’s lifecycle.
2. How does AI improve pharma documentation?
AI automates data extraction, improves accuracy, accelerates approvals, and ensures compliance with evolving regulations.
3. Can eZintegrations™ handle FDA regulatory submissions?
Yes, it can integrate with eCTD systems and route documents to meet FDA compliance.
4. Is Goldfinch AI trained specifically for life sciences?
Yes, its NLP models are fine-tuned for pharma-specific language and document types.
5. How secure is AI-based pharma document management?
Platforms like eZintegrations™ support enterprise-grade encryption, access control, and full audit trails.