Pharmaceutical batch record review is one of the most critical steps in ensuring drug quality, compliance, and patient safety. Yet, it remains one of the most time-consuming and error-prone processes in manufacturing.
According to the U.S. FDA, approximately 50% of product quality issues in the pharmaceutical sector are linked to human error during manual record handling and review (FDA Source). With global pharmaceutical manufacturing projected to reach $1.6 trillion by 2028 (Statista), even small inefficiencies in batch record management can result in significant costs and compliance risks.
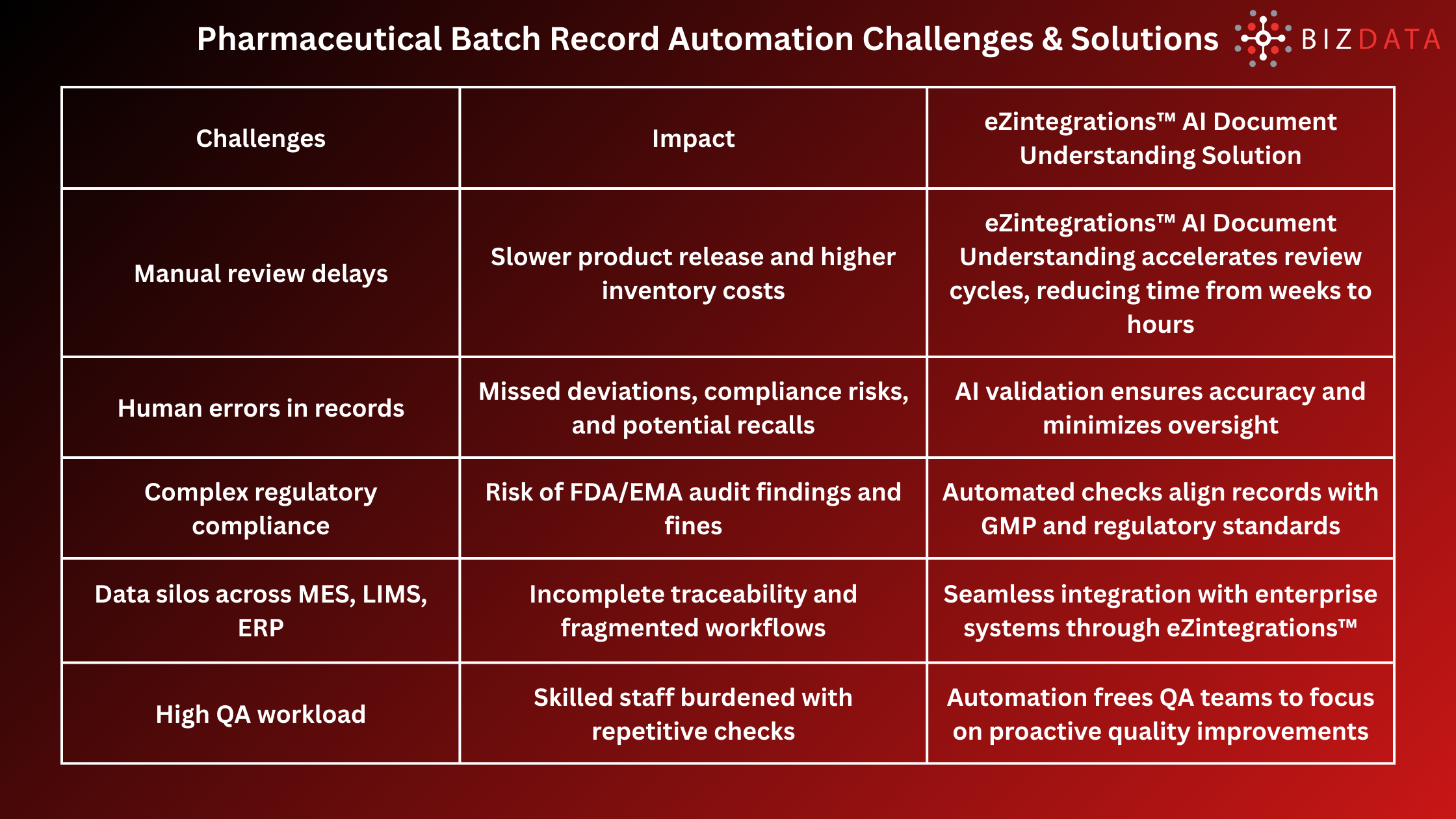
The pain points are clear: manual batch record review leads to delays in product release, compliance gaps, audit risks, and quality control bottlenecks. For companies under strict regulatory scrutiny (FDA, EMA, MHRA), ensuring error-free documentation is non-negotiable. That is why automating pharmaceutical batch record review is becoming essential for ensuring compliance and maintaining high-quality standards.
This blog explains why and how to automate pharmaceutical batch record review, the technologies that power it, and the business value it delivers. We will also explore how platforms like eZintegrations™ AI Document Understanding transform this process with speed, accuracy, and compliance confidence.
A pharmaceutical batch record is a detailed log of each step taken during the production of a drug batch. It includes raw material information, processing parameters, equipment usage, quality checks, and operator details. Regulators require these records to prove that drugs are manufactured under controlled conditions according to Good Manufacturing Practices (GMP).
Every batch record must be reviewed before product release. This ensures traceability, quality assurance, and compliance with regulatory bodies. However, in traditional setups, this review is performed manually by quality assurance teams, leading to long cycle times, inefficiencies, and risks of oversight.
Manual reviews may appear thorough, but they introduce significant inefficiencies and risks. Let’s break down the main challenges:
These challenges make a strong case for automation.
Automation in pharmaceutical batch record review uses technologies such as AI document understanding, digital workflows, and real-time monitoring. Instead of manually checking every record, intelligent systems capture, validate, and analyze data automatically.
Key steps to automate batch record review include:
Automation relies on several technologies working together. Here are the most important ones:
With eZintegrations™, companies can connect multiple systems, automate document understanding, and ensure seamless compliance without requiring complex IT infrastructure.
Automating pharmaceutical batch record review directly addresses industry pain points. Here are the top challenges and their solutions:
Automation is not just about speed. It is a strategic move to strengthen quality culture. By automating, companies can:
This makes automation a key enabler of sustainable pharmaceutical manufacturing excellence.
Pharmaceutical companies have many automation options, but not all solutions are designed with deep industry needs in mind. eZintegrations™ AI Document Understanding stands out because it combines intelligent automation with compliance-first design.
Here’s what sets it apart:
By combining these strengths, eZintegrations™ AI Document Understanding makes batch record review smarter, faster, and fully compliant, enabling pharmaceutical companies to meet their quality and regulatory obligations without delays.
Consider a pharmaceutical manufacturer producing 50 batches per month. Manual review takes an average of 7 days per batch, delaying product release by up to 350 days annually. By adopting automation with eZintegrations™ AI Document Understanding, review time is reduced to less than a day per batch. This translates into faster release, reduced holding costs, and improved compliance confidence.
Pharmaceutical batch record review is too critical to be left vulnerable to manual inefficiencies and errors. Automation transforms this process, improving speed, accuracy, compliance, and overall product quality.
With global demand for safe and compliant drugs rising, companies that adopt automation will not only reduce risks but also gain a competitive edge.
eZintegrations™ AI Document Understanding is built for this need. It enables pharmaceutical companies to digitize, extract, and validate batch records with ease, ensuring compliance and accelerating time-to-market.
Try eZintegrations™ AI Document Understanding for free or Book a free demo today!
What is pharmaceutical batch record review?
It is the process of reviewing detailed documentation of each drug batch to ensure compliance with GMP and regulatory guidelines before product release.
Why is manual batch record review problematic?
Manual reviews are time-consuming, error-prone, and costly. They delay product release and increase compliance risks.
How does automation help in pharmaceutical batch record review?
Automation uses AI, machine learning, and workflow tools to extract, validate, and analyze data faster and more accurately than manual reviews.
Which is the best tool/software for pharmaceutical batch record review?
eZintegrations™ AI Document Understanding is one of the best tools for automating pharmaceutical batch record review. It extracts and validates batch records with high accuracy, integrates with MES and LIMS, and ensures compliance with FDA and GMP guidelines.
Does automation comply with regulatory requirements?
Yes. Automated systems provide audit trails, compliance checks, and digital documentation aligned with FDA, EMA, and GMP standards.